Prime Minister’s Research Fellowship (PMRF)

Reshma Babu
Academic background:
Research Interest:
My current research work is mainly focused on the novel homogeneous catalytic design and development for acceptorless (de)hydrogenation reactions and valuable sustainable chemical synthesis. Rapid depletion of fossil fuels and growing environmental concerns directed chemists and chemical industries to identify alternative renewable energy resources. It has been widely accepted that hydrogen as a fuel can be effective to curtail this energy crisis. In this context, acceptorless dehydrogenation reactions in which H2 is liberated from abundant, renewable feedstocks and new bonds generated by further reactions of the dehydrogenated product are emerging as a powerful approach. Acceptorless alcohol dehydrogenation by homogeneous transition-metal catalysis represents a powerful and sustainable route for synthetic purposes as well as for energy production. In this regard, the design and development of new catalytic systems for fundamentally important synthetic transformations and energy storage applications is an intellectually stimulating challenge. Hence, my research works are mainly focusing on this area.
Advisor information:
Dr. Ekambaram Balaraman, a chemist studying transition-metal catalysis as well as an enthusiastic chemistry teacher has received a Ph.D. degree in chemistry from the University of Hyderabad and Postdoctoral research from Weizmann Institute of Science, Israel. He has worked as a senior scientist (July 2013-Dec 2018) at CSIR-NCL, Pune. He has received numerous awards and honours including SwarnaJayanti Fellowship (2020), Bronze Medal from the Chemical Research Society of India (CRSI) – 2020, Thieme Journal Award (2020), The Asian and Oceanian Photochemistry Association (APA) for Young Scientist (2019), AV Rama Rao (AVRA) Young Scientist Award (2018). He is an elected member of The National Academy of Sciences, India (2020), Fellow of Royal Society of Chemistry (FRSC), Affiliate Member of International Union of Pure and Applied Chemistry (IUPAC), Member of the Indian Nation Young Academy of Science (INYAS), INSA (2018-2022), Life member of Catalysis Society of India, Member of American Chemical Society (2010), Member of Israel Chemical Society.
Dr. E. Balaraman’s research has mainly focused on catalyst (Homogeneous & Heterogeneous) development for dehydrogenation reactions, hydrogen auto-transfer reactions, C1-chemistry (CO2 to value-added chemicals), green chemistry by heterogeneous catalysis, and Ziegler-Natta catalysis. A highlight of his research involves the use of pincer ligands, particularly those with substituents that absorb or release molecular hydrogen. Also, he examined the activation of C-C, C-H, N-H and O-H bonds. His main research involves the design and development of new catalytic systems for energy and environmental applications. Dr. E. Balaraman had significantly contributed to catalyst design for various important reactions for both fundamental research and industrial application. He has also contributed to heterogeneous acceptorless dehydrogenation and related reactions based on developed nanocatalysts. for CO2 utilization and its direct application as a renewable raw material for polymer synthesis.
WRITE-UP FOR PMR FELLOWSHIP FROM NOV 2021 – PRESENT
PhD Topic: 3d- Transition Metal Catalyzed Activation of Alcohols: Applications in Acceptorless Alcohol Dehydrogenation and Related Reactions
Chapter 1
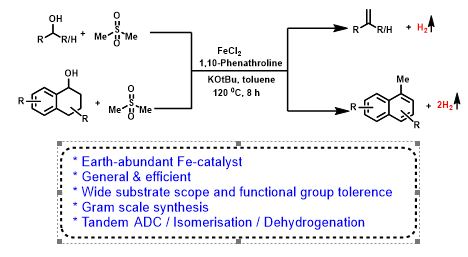
Iron Catalyzed Direct Julia-Type Olefination of Alcohols
Olefins are valuable for synthetic chemistry and ubiquitous in polymer, agrochemical, pharmaceutical, and functional materials. Herein, we have carried out the first iron-catalyzed convenient, and expedient strategy for the synthesis of styrene and naphthalene derivatives with the liberation of dihydrogen. The present olefination has a broad substrate scope with primary and secondary alcohols. Interestingly, unprecedented synthesis of 1-methyl naphthalenes proceeds via tandem methenylation/double dehydrogenation was reported.
Chapter 2:
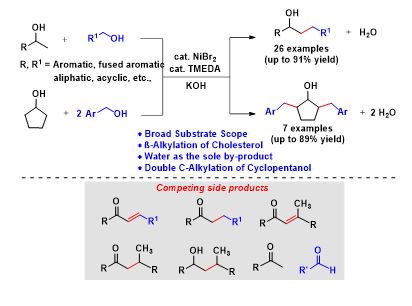
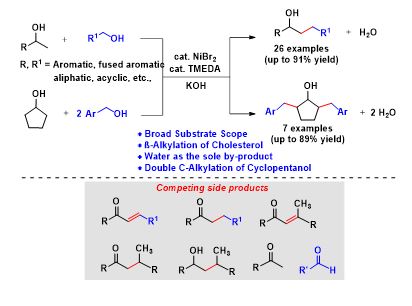
Nickel-Catalyzed Guerbet Type Reaction: C-Alkylation of Secondary Alcohols via Double (de)hydrogenation
The construction of functionalized diverse molecules from simple abundant chemicals by C-C bond-forming reactions is one of the fundamental reactions in organic synthesis. Herein, we have done the selective β-alkylation of secondary alcohols with primary alcohols, using a commercially available, inexpensive NiBr2/TMEDA as an efficient catalytic system through acceptorless double dehydrogenative cross-coupling strategy. Interestingly, more challenging substrate conversions were achieved under benign conditions using primary alcohols as potential alkylating agents with various secondary alcohols. A broad range of substrates including aromatic, cyclic, acyclic, and aliphatic alcohols was well tolerated. Interestingly, the C-alkylation of cholesterol derivatives and the double C-alkylation of cyclopentanol with various alcohols were also demonstrated.
Chapter 3
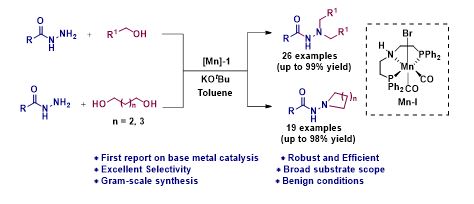
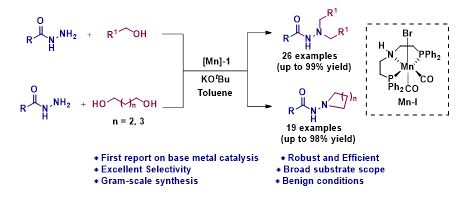
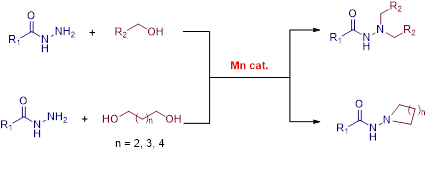
Manganese Catalyzed Double Borrowing Hydrogenation: N,N-Dialkylation and Cyclization of Acylhydrazides Using Alcohols
N, N-disubstituted acyl hydrazide and its derivatives are privileged synthetic scaffolds that have been widely used for the synthesis of agrochemicals, polymers, pharmaceuticals, and bio-active functional materials. Herein, we report the first earth-abundant base metal catalyzed direct one-pot N,N-dialkylation and cyclization of acylhydrazides using alcohols. The reaction is catalyzed by a well defined Mn(1)-based PNP-pincer complex and it operates via the borrowing hydrogen strategy. Surprisingly, more challenging substrates were tolerated under standard reaction condition using alcohols as potential alkylating agents. Interestingly, really demanding cyclization of acylhydrazides with diols also demonstrated.
Chapter 4
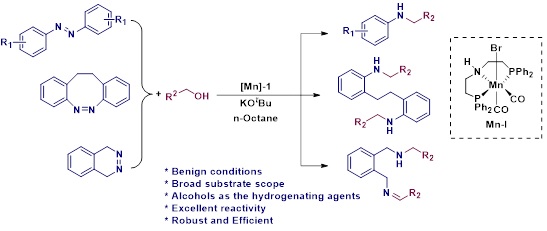
Efficient Manganese Catalyzed Unprecedented Hydrogenation and N-alkylation of Azo (N=N) Bonds to Alkylated Amines Using Alcohols
N-alkylated amine derivatives have wide spread applications in agrochemicals, pharmaceuticals, lubricants, organic dyes, corrosion inhibitors, surfactants and polymer industry. Herein, we systematically explore complete hydrogenation and N-alkylation of azo (N=N) bonds to alkylated amines by using a well-defined Mn-PNP pincer complex via metal-ligand incorporation strategy. Interestingly, the reaction involves abundantly available renewable feedstocks such as alcohols, that can act as hydrogenating as well as the alkylating agents which makes this present work an eco-friendly, atom-economical, step-economical and sustainable approach for alkylated amine synthesis. Moreover, really demanding and more challenging N-alkylation of cyclic diazocines also performed.
External Teaching Assistantships
- Coordination Chemistry (At Sri Venkateshwara College of Engineering, TIRUPATI)
Achievements
- Academic Excellence Award
- Delivered a talk on Mole Day symposium, 23rd October 2021 at IISER-Tirupati.
- Qualified PMRF review held on 28th December 2021 with ‘very good’ grade.
Publications
- Rana, R. Babu, M. Subaramanian and E. Balaraman*, Ni-catalyzeddehydrogenative coupling of primary and secondary alcohols with methyl-N-heteroaromatics, Org. Chem. Front., 2018, 5, 3250–3255.
- Landge, R. Babu, V. Yadav, M. Subramanian, Gupta, V, and E. Balaraman*, Iron-Catalyzed Direct Julia-type Olefination of Alcohols, J. Org, Chem., 2020, 85, 9876−9886.
- Babu, M. Subaramanian, S. P. Midya and E. Balaraman*, Nickel-catalyzed Guerbet type reaction: C-alkylation of secondary alcohols via double (de)hydrogenation, Org. Lett., 2021, 23, 3320–3325.
- P. Midya, M. Subaramanian, R. Babu, V. Yadav, and E. Balaraman*, Tandem acceptorless dehydrogenative coupling-decyanation under nickel catalysis, J. Org. Chem. 2021, 86, 7552-7562.
Papers in preparation
- Babu, G. Sivakumar and E. Balaraman*, Manganese Catalyzed Double Borrowing Hydrogenation: N,N-Dialkylation and Cyclization of Acylhydrazides Using Alcohols (Submitted).
- Babu, E. Balaraman*, Efficient Manganese Catalyzed Unprecedented Hydrogenation and N-alkylation of Azo (N=N) Bonds to Alkylated Amines Using Alcohols (Manuscript under preparation).
WRITE-UP FOR PMR FELLOWSHIP FROM FEB 2021 – NOV 2021
Academic Progress:
- Open Seminar (the first stage of Ph. D.):
- Tittle: Recent Advances in Catalytic Acceptorless Alcohol Dehydrogenation Reactions: Role of Pincer Ligands
- Date: 11-02-21
The presentation was rated as excellent by the committee members.
- Comprehensive exam (the second stage of Ph. D.):
- Date: 22-03-21
The viva-voce examination was highly appreciated and approved.
- RAC-1 (the third stage of Ph. D.)
- Tittle: 3d-Transition Metal Catalyzed Activation of Alcohols: Applications in Acceptorless Alcohol Dehydrogenation and Related Reactions
- Date: 21-05-21
- RAC Members:
Prof. Ramakrishna G. Bhat (Chemistry, IISER-Pune)
Dr. Sudipta Roy (Chemistry, IISER-Tirupati)
Dr. Kiran Kumar (Chemistry, IISER-Tirupati)The presentation was good and RAC committee recommended JRF to SRF upgradation.
- First RAC’s presentation involved three chapters:
Chapter 1:
Iron Catalyzed Direct Julia-Type Olefination of Alcohols
Olefins are valuable for synthetic chemistry and ubiquitous in polymer, agrochemical, pharmaceutical, and functional materials. Herein, we have carried out the first iron-catalyzed convenient, and expedient strategy for the synthesis of styrene and naphthalene derivatives with the liberation of dihydrogen. The present olefination has a broad substrate scope with primary and secondary alcohols. Interestingly, unprecedented synthesis of 1-methyl naphthalenes proceeds via tandem methenylation/double dehydrogenation was reported.
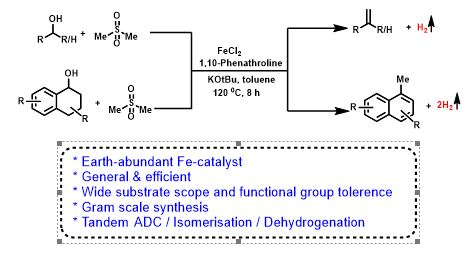
Chapter 2:
Nickel-Catalyzed Guerbet Type Reaction: C-Alkylation of Secondary Alcohols via Double (de)hydrogenation
The construction of functionalized diverse molecules from simple abundant chemicals by C‒C bond-forming reactions is one of the fundamental reactions in organic synthesis. Herein, we have done the selective β-alkylation of secondary alcohols with primary alcohols, using a commercially available, inexpensive NiBr2/TMEDA as an efficient catalytic system through acceptorless double dehydrogenative cross-coupling strategy. Interestingly, more challenging substrate conversions were achieved under benign conditions using primary alcohols as potential alkylating agents with various secondary alcohols. A broad range of substrates including aromatic, cyclic, acyclic, and aliphatic alcohols was well tolerated. Interestingly, the C-alkylation of cholesterol derivatives and the double C-alkylation of cyclopentanol with various alcohols were also demonstrated.
Chapter 3:
Manganese Catalyzed Double Borrowing Hydrogenation: N,N-Dialkylation and Cyclization of Acylhydrazides Using Alcohols
The construction of functionalized diverse molecules from simple abundant chemicals by C‒C bond-forming reactions is one of the fundamental reactions in organic synthesis. Herein, we have done the selective β-alkylation of secondary alcohols with primary alcohols, using a commercially available, inexpensive NiBr2/TMEDA as an efficient catalytic system through acceptorless double dehydrogenative cross-coupling strategy. Interestingly, more challenging substrate conversions were achieved under benign conditions using primary alcohols as potential alkylating agents with various secondary alcohols. A broad range of substrates including aromatic, cyclic, acyclic, and aliphatic alcohols was well tolerated. Interestingly, the C-alkylation of cholesterol derivatives and the double C-alkylation of cyclopentanol with various alcohols were also demonstrated.
External Teaching Assistantships:
- Coordination Chemistry and Spectroscopy (at Sri Venkateshwara College of Engineering, Tirupati)
Achievements:
- Academic Excellence Award
- Delivered a talk on Mole Day symposium, 23rd October 2021 at IISER-Tirupati.
Publications
- J. Rana, R. Babu, M. Subaramanian and E. Balaraman*, Ni-catalyzed dehydrogenative coupling of primary and secondary alcohols with methyl-N-heteroaromatics, Org. Chem. Front., 2018, 5, 3250–3255.
- V. Landge, R. Babu, V. Yadav, M. Subramanian, Gupta, V, and E. Balaraman*, Iron-Catalyzed Direct Julia-type Olefination of Alcohols, J. Org, Chem., 2020, 85, 9876−9886.
- R. Babu, M. Subaramanian, S. P. Midya and E. Balaraman*, Nickel-catalyzed Guerbet type reaction: C-alkylation of secondary alcohols via double (de)hydrogenation, Org. Lett., 2021, 23, 3320–3325.
- S. P. Midya, M. Subaramanian, R. Babu, V. Yadav and E. Balaraman*, Tandem acceptorless dehydrogenative coupling-decyanation under nickel catalysis, J. Org. Chem. 2021, 86, 7552-7562.
Papers in preparation
- R. Babu, G. Sivakumar and E. Balaraman*, Selective N,N-dialkylation and cyclization of acylhydrazides using alcohols under manganese catalyzed double borrowing hydrogenation conditions
WRITE-UP FOR PMR FELLOWSHIP FROM AUG 2020 – FEB 2021
Courses done during PhD:
- Physical Organic Chemistry
- Bio-inorganic Chemistry
- Transition-Metal chemistry
- Organic Synthesis 1
Teaching assistantships:
- CHM 122 – Chemistry Laboratory 1
- CHM 211 –Inorganic Chemistry
Objectives of my thesis:
Chapter 1.
Nickel-Catalyzed β-Alkylation of Secondary Alcohols with Primary Alcohols via Borrowing Hydrogen Strategy
We have studied a catalytic cross-coupling of primary and secondary alcohols via borrowing hydrogen strategy under nickel catalysis. This method provides an atom-economical method for β-alkylation of secondary alcohols under mild, benign conditions. Interestingly the β-alkylation of cholesterol derivative and double β-alkylation of cyclopentanol with various alcohols were also demonstrated.
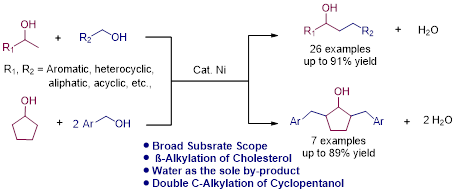
Chapter 2.
Manganese Catalyzed Direct Catalytic Symmetrical, Unsymmetrical N,N-Dialkylation and Cyclization of Acylhydrazides Using Alcohols.
Our Mn-catalytic protocol provides a one-pot synthesis of both symmetrical and unsymmetrical N,N-disubstitutedacylhydrazides using alcohols. Interestingly, the use of diols resulted in the intermolecular cyclization of acylhydrazides and such products are more relevant in biologically active compounds.
Publications
- J. Rana, R. Babu, M. Subaramanianand E. Balaraman*, Ni-catalyzeddehydrogenative coupling of primary and secondary alcohols with methyl-N-heteroaromatics, Org. Chem. Front., 2018, 5, 3250–3255.
- V. Landge, R. Babu, V. Yadav, M. Subramanian, Gupta, V, and E. Balaraman*, Iron-Catalyzed Direct Julia-type Olefination of Alcohols, J. Org, Chem., 2020, 85, 9876−9886.


